A Guide to Car Battery Types
From basic to high end, here's a guide to how automotive batteries work, their pros and cons, and how much they cost.
 General Motors
General Motors
Every car battery performs the same primary functions — starting your car and powering its accessories. When it comes time to replace your vehicle's battery, there are several types to choose from, depending on the kind of vehicle you have. From the least expensive and most basic lead-acid batteries to high-end lithium-ion batteries, here is what you need to know.
Lead-Acid Batteries
Lead-acid batteries emerged in the 1910s to power the electric starter motor that became increasingly common during the early days of the automobile. The battery-powered self-starting system made firing up a car much safer and easier compared to manual hand cranks. Like many of the industry's innovations, the car battery appeared in more expensive models before trickling into affordable cars such as General Motors fleets. The revolutionary Ford Model T played a significant role in democratizing the car across the United States but didn't come standard with a battery until 1919.
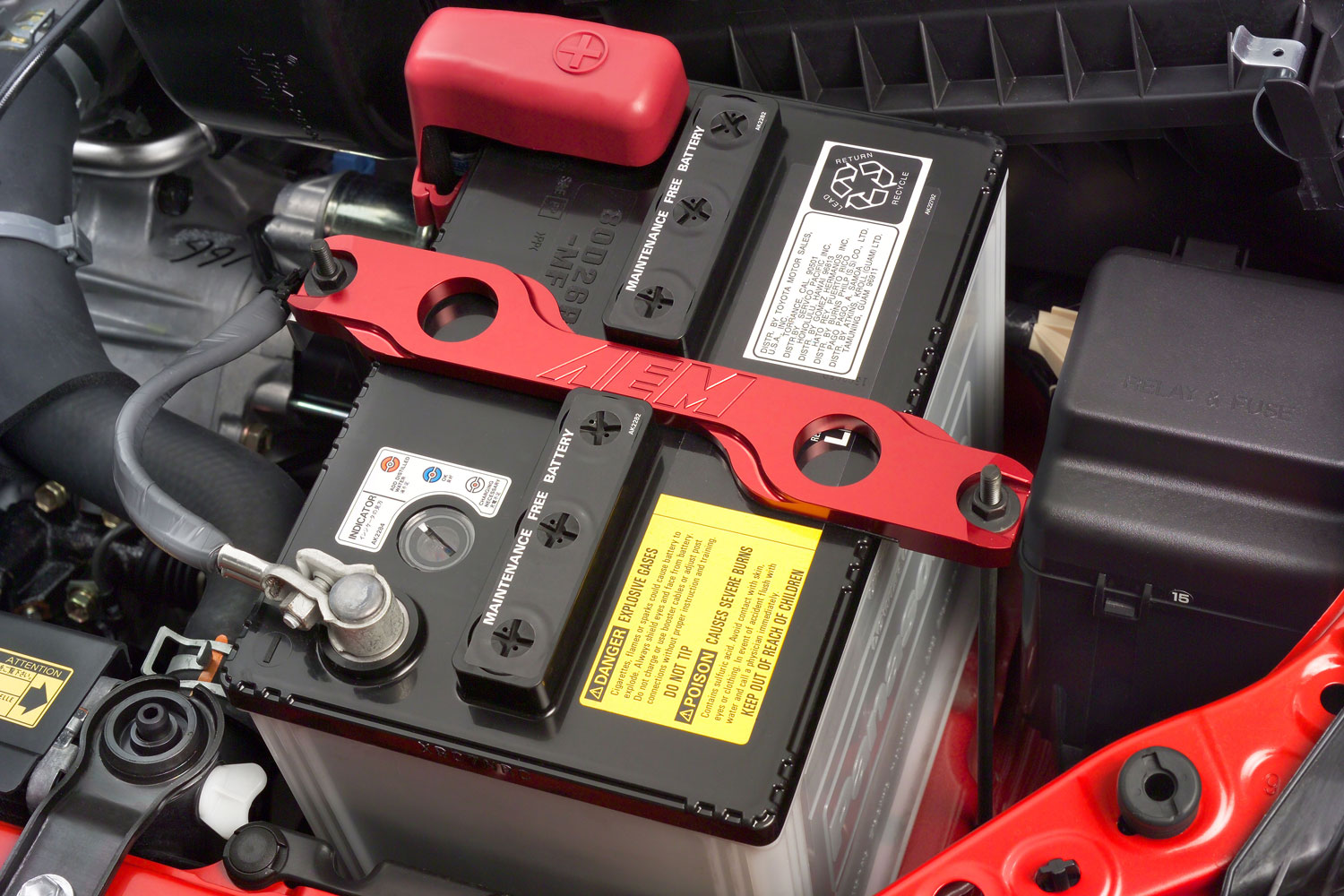
Lead acid refers to how this battery produces electricity. Most modern car batteries contain six cells, each housing a lead-dioxide plate and a lead plate. The plates are immersed in sulfuric acid, and together they create a chemical reaction that produces ions and lead sulfate. In turn, the ions have hydrogen and lead sulfate, and this reaction creates the electrons required to start your car's engine.
Lead-acid batteries typically cost less than other types of batteries, such as lithium-ion batteries. However, factors like size and cold-cranking amps affect pricing. They're also heavier than other battery types.
 Toyota
Toyota
AGM Batteries
First used in the 1970s, absorbent glass mat (AGM) batteries use an evolution of lead-acid technology. They rely on malleable glass mats positioned between the lead plates to produce and store more energy, so they're better suited for heavy-duty applications such as modern cars with numerous electronics, such as the Toyota Prius. (This is not the high-voltage hybrid battery but the smaller 12-volt found in the engine bay.)
An AGM battery typically lasts longer than a comparable lead-acid battery, but it also costs more. The glass mats also help prevent potentially dangerous spills by absorbing the sulfuric acid.
Gel Cell Batteries
More common in powersports vehicles such as ATVs, motorcycles, and personal watercraft, gel cell batteries use the same basic technology as lead-acid and AGM batteries but rely on a compound called silica — also found in sand — to turn the sulfuric acid into a gel. This makes the battery spill-proof, so it works well in off-road applications.
Gel cell batteries cost more than comparable AGM batteries, which are also spill-proof. They are also sensitive to quick charges and discharges, so car gel cell batteries are relatively rare.
 Toyota
Toyota
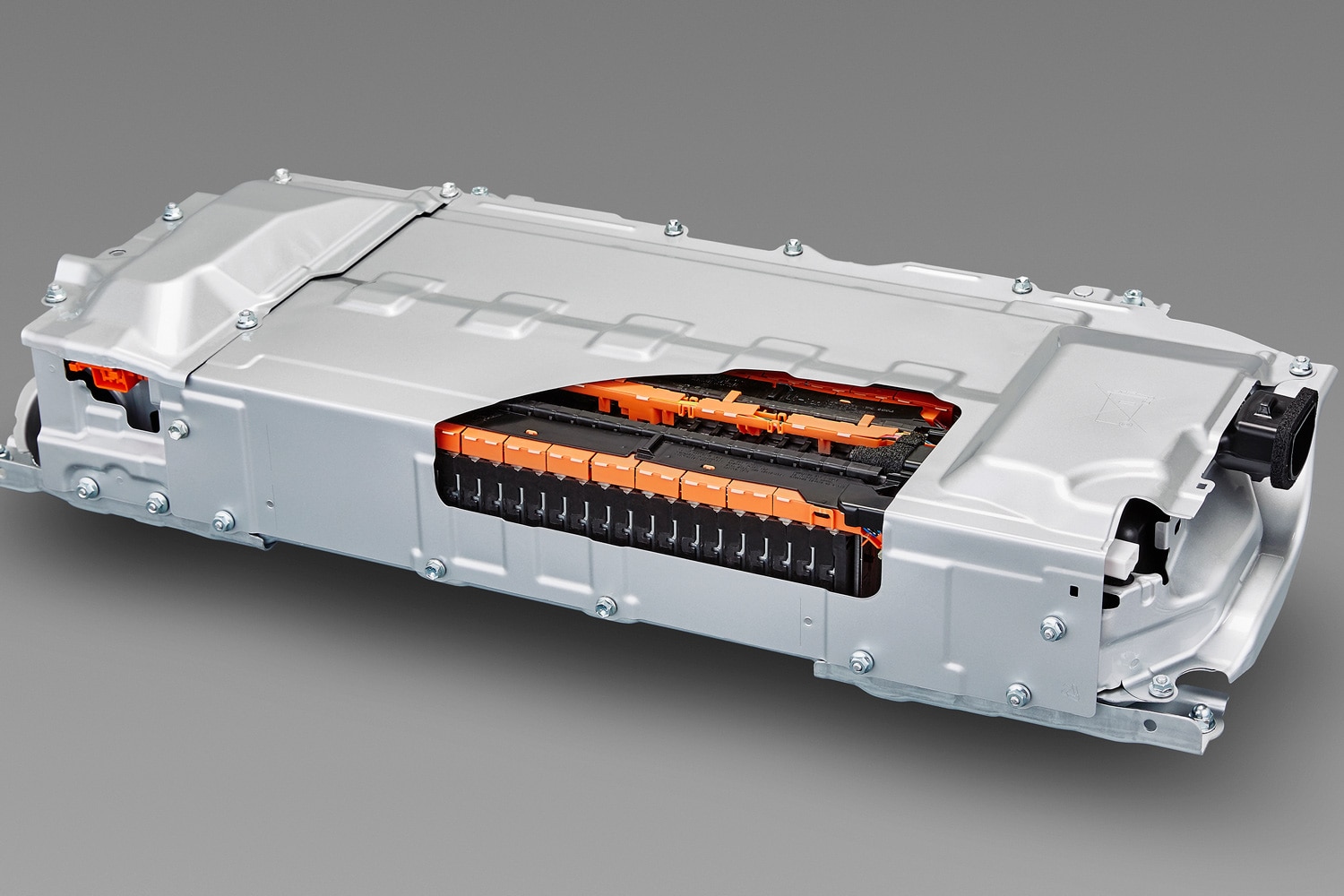
Lithium-Ion Batteries
Lithium-ion batteries power the majority of modern electric vehicles, such as the Toyota bZ4X. This technology also powers some 12-volt car batteries. The conductive liquid in these batteries contains lithium ions instead of lead ions in a lead-acid battery. This chemistry creates electricity by letting the electrons move between the positive and negative electrodes via the part receiving power, like an engine's starter motor or an electric vehicle's drive motor.
One of the main advantages of lithium-ion batteries is their high energy density, meaning they're lighter and smaller than a comparable lead-acid battery. However, they're prone to overheating, don't last as long as other types of batteries, and are more expensive.
Written by humans.
Edited by humans.
 Ronan Glon
Ronan GlonRonan Glon is an American journalist and automotive historian based in France. He enjoys working on old cars and spending time outdoors seeking out his next project car.
Related articles
View more related articles